Required components for CRISPR-Cas9 gene editing and synthesis of guide RNA
Sequence structure of a synthetic sgRNA
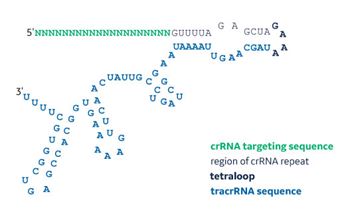
The CRISPR-Cas9 system allows researchers to quickly edit genes for functional protein knockout in mammalian, fish and plant genomes, among others, and consequently has dramatically transformed biological research. The natural Streptoccocus pyogenes CRISPR-Cas9 system requires three components: 1. Cas9 nuclease, 2. CRISPR RNA (crRNA) comprised of targeting and repeat sequences, and 3. tracrRNA, which hybridizes to the crRNA through its anti-repeat sequence. The crRNA:tracrRNA complex recruits the Cas9 nuclease and cleaves DNA upstream of a protospacer-adjacent motif (PAM). The crRNA and tracrRNA can be linked together with a loop sequence for generation of a chimeric single guide RNA (sgRNA; Hsu et al. 2013). sgRNA can be generated for DNA-based expression or by chemical synthesis. Using patented Dharmacon 2'-ACE chemistry (Scaringe et al. 1998, Scaringe et al. 2004), long RNA can be routinely synthesized with faster coupling rates, higher yields and greater purity and is ideal for generating synthetic sgRNAs.
Benefits of synthetic sgRNA in gene editingAlthough the synthetic two-part RNA (crRNA:tracrRNA) system is efficient and cost-effective for most applications, researchers working with in vivo and ex vivo models have indicated a preference for a sgRNA system. The advantages to using a synthetic sgRNA compared to plasmid-expressed or in vitro transcribed sgRNA include:
- A single oligonucleotide, arrives ready to use
- No cloning, sequencing, or in vitro transcription required
- Options for completely DNA-free gene editing when combined with Cas9 mRNA or Cas9 protein
- Allows for incorporation of chemical modifications
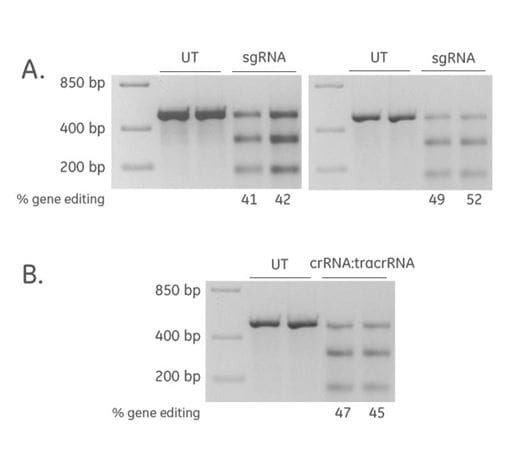
Efficient CRISPR-Cas9 gene editing achieved with synthetic sgRNA and synthetic crRNA:tracrRNA
2'-ACE chemistry was used to synthesize a sgRNA (Hsu et al. 2013, Briner et al. 2014) targeting PPIB, which was then purified by HPLC. A U2OS cell line stably expressing Cas9 nuclease from the CAG promoter was plated at 10,000 cells per well in 96-well format one day prior to transfection. sgRNA (25 nM) or synthetic crRNA:tracrRNA (25 nM) was transfected into duplicate wells using DharmaFECT™ 3 transfection reagent (0.25 μL/well). After 72 hours, direct cell lysis was amplified using primers surrounding the target site on the PPIB gene and gene editing efficiency estimated using a mismatch detection assay (Edit-R™ Synthetic crRNA Positive Controls - Protocol). The synthetic sgRNA for target gene editing resulted in high editing efficiency (data shown are from two experiments; (A), and high editing efficiency was also achieved for synthetic crRNA:tracrRNA (B).
References
- Briner, A.E., Donohoue, P.D., et al. , Guide RNA functional modules direct Cas9 activity and orthogonality. Mol Cell. 56(2), 333-339 (2014).
- Hsu, P.D., Scott, D.A., et al., DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31(9), 827-832 (2013).
- Hendel, A, et. al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat.Biotechnol. 33, 985-989 (2015).
- Scaringe, S.A. et al., Novel RNA synthesis method using 5´-silyl-2´-orthoester protecting groups. J. Am. Chem. Soc. 120:11820-11821 (1998).
- Scaringe, S.A. et al., Preparation of 5´-silyl-2´-orthoester ribonucleosides for use in oligoribonucleotide synthesis. Curr. Protoc. Nuc. Acid Chem. 2.10.1-2.10.16 (2004).
Order Products
Edit-R CRISPR synthetic sgRNA
Algorithm designed synthetic sgRNA guaranteed to edit the target gene of interest for accurate and efficient gene knockout.
CRISPR Design Tool
Design and order custom synthetic sgRNA in tubes or 96-well plates.
Cas9 nuclease
Configure the optimal promoter for your cell type to ensure robust Cas9 expression or explore DNA-free option
CRISPR controls and detection primers
Proper controls are essential to assessment of CRISPR-Cas9 genomic editing experiments
Helpful Resources
Edit-R CRISPR-Cas9 Gene Engineering Platform - Video
Learn how to choose the right products for your CRISPR gene knockout workflow, cell type(s) and experimental goals. This video walks through a few high-level questions to help direct you to the best components for your experiment.
View poster
Chemical Synthesis of Long and Highly Modified RNA using 2'-ACE Chemistry
