The considerations around resistance vs sensitivity screens and the appropriate modalities for each
Positive Selection Screening: Resistance screens
If the researchers’ aim is to find resistance hits, i.e., genes that when knocked out (CRISPRko), knocked down (CRISPRi) or overexpressed (CRISPRa) create a phenotype with improved cellular fitness that will become enriched as the resistance screen progresses, we apply a high drug pressure, at a near-lethal dose, to create the appropriate growth inhibition conditions, ideally between 70-90% (Figure 1). This will ensure that cells harboring a certain perturbation conferring drug resistance are more likely to survive and therefore that phenotype will be enriched in the total cell population relative to more sensitive phenotypes which become depleted. This would be detected during NGS analysis by comparison between the drug-treated and control end-point cell populations.
Resistance screens can be utilized throughout drug-development pipelines, including the identification of markers to stratify patients based on their genetic profile, that would place them in the appropriate treatment groups. Additionally, identification of resistance genes can aid in the generation of combinatorial treatment strategies that would overcome resistant phenotypes.
Negative Selection Screening: Sensitivity screens
If the researchers’ aim is to find sensitivity hits, i.e., genes that when knocked out (CRISPRko), knocked down (CRISPRi) or overexpressed (CRISPRa) create a phenotype with reduced cellular fitness, that will be depleted as the sensitivity screen progresses, we must apply a moderately low drug pressure, to create a growth inhibition around 10-30%. This is because in a sensitivity or negative selection screen, we aim to find a phenotype that is being lost in the population, due to its increased sensitivity to the effects of the drug being used.1 By applying a consistently small drug pressure, we have an ideal assay window for sensitivity screens (Figure 1) as it provides confidence of target engagement by having a measurable impact on growth inhibition, whilst providing a low drug pressure to identify only the perturbations that are the most sensitive to the drug.
In a sensitivity or negative selection screen, to improve screen quality, we can benefit from choosing a library with higher number of guides per gene1 to provide a more statistically robust experimental set up for interrogation depth.
During drug-development pipelines, sensitivity screens can be helpful to identify gene perturbations that sensitize resistant cells to the effects of a drug, thus suggesting synergies between multiple therapeutic strategies and also aid in the identification of patients who would benefit from a selected treatment.
Other considerations
To identify effective negative- or positive-screen doses it is vitally important to perform a pre-screen evaluation, performing a thorough investigation of multi -dosing regimens (multiple doses, over extended time periods) with the compound of interest to identify an appropriate screen pressure, and understanding of the cell line response over time.
When performing a sensitivity screen, the use of 10-30% growth inhibition recommended to identify genetic perturbations sensitive to the drug can potentially enable the identification of early onset resistance hits, albeit with lower confidence than when a screen performed at the appropriate drug pressure for a resistance screen (growth inhibition around 70-90%). These additional resistance hits from a sensitivity screen may often confirm the drug effect on a known target (by knocking out the drug target one would expect resistance to the drug would start to develop).
The opposite situation, analyzing sensitivity hits from a resistance screen is not usually as informative as the elevated drug pressure used to cause resistance conditions conflicts with obtaining the optimal window for the detection of genes dropping out from the population (Figure 1).
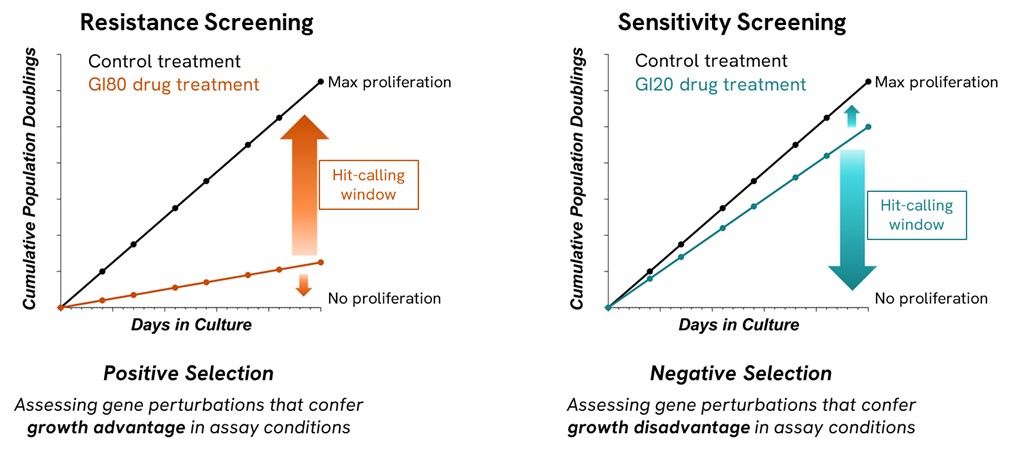 Figure 1 - In a positive selection screen we look for the enrichment of genes resistant to the drug when there is high drug pressure (70-90% GI), this creates the optimal window to identify resistance hits. In a negative selection screen, we use a lower drug pressure 10-30% to maximize the window for the detection of genes dropping out from the cell population.
Figure 1 - In a positive selection screen we look for the enrichment of genes resistant to the drug when there is high drug pressure (70-90% GI), this creates the optimal window to identify resistance hits. In a negative selection screen, we use a lower drug pressure 10-30% to maximize the window for the detection of genes dropping out from the cell population.
References
- Blanck M, Budnik-Zawilska MB, Lenger SR, McGonigle JE, Martin GRA, le Sage C, Lawo S, Pemberton HN, Tiwana GS, Sorrell DA, Cross BCS. A Flexible, Pooled CRISPR Library for Drug Development Screens. CRISPR J. 2020 Jun;3(3):211-222. https://doi.org/10.1089/crispr.2019.0066

