How do functional genomic and cell panel screening combine to identify novel targets?
Functional Genomic Screening (FGS) and Cell Panel Screening (CPS) can both provide comprehensive data sets to support and drive forward therapeutic development. They provide orthogonal approaches to the researcher. Whilst both approaches have unique strengths, when utilized in combination they can identify novel targets, cross-validated by independent approaches.
Why use functional genomic screening?
FGS employs genetic perturbagens (e.g. CRISPRko, CRISPRi, CRISPRa, siRNA, ASOs) to allow the researcher to scrutinize genetic interactions. When performed in combination with chemical perturbagens, these screens can probe drug-gene interactions to:
• Identify gene drivers for sensitivity or resistance phenotypes.
• Identify the involvement of cellular pathways.
• Provide understanding of drug mechanism of action (MOA).
FGS can be performed in both pooled and arrayed formats from small scale through to whole genome, with the choice driven by the screen parameters, including: assay type, cells utilized, scale, reagent type, and endpoint. Pooled screening is an excellent choice for larger, proliferation or selection-based screens. Arrayed FGS provides the option for multiple phenotypic read-outs for an in-depth exploration using defined genetic (and chemical) perturbagens, including using co-culture assay formats.
Why use cell panel screening?
The high-throughput approach of CPS allows the assessment of chemical perturbagens in multiple cellular backgrounds and tissue types. By quantitating phenotypic response, CPS can highlight cell lines or tissues that showed enhanced sensitivity or resistance to the test agent allowing the researcher to hone their treatment strategy and understand clinical therapeutic potential. Further versatility can be gained by combinational therapeutic screening (including with standard of care drugs) to explore for synergistic relationships.
Combining functional genomic and cell panel screening
To showcase how FGS and CPS, both versatile tools that can be leveraged throughout the therapeutic development pipeline (Figure 1), can be utilised together to provide direct validation of screen hits we performed a pooled CRISPR knockout (CRISPRko) screen using the PARP inhibitor, Olaparib, followed by different CPS screening formats (Blanck et al., 2020; Blanck, 2023)
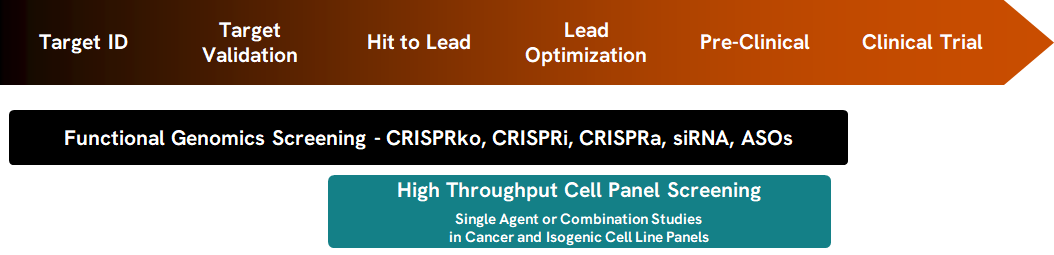
Figure 1. Functional Genomic Screening and Cell Panel Screening can support multiple phases of the therapeutic development pipeline.
A negative selection pooled CRISPRko screen was performed to identify gene knockouts that show increased sensitivity to the therapeutic treatment and are detected by a depletion in abundancy of these knockout genotypes within the cell pool. The top sensitivity hits identified in the pooled CRISPRko screen following PARP inhibition, included ATM, FANC pathway members and the RNaseH2 complex, and showed good agreement with published CRISPRko PARP inhibitor screens performed in other cell lines (Zimmerman et al., 2018).
To profile cell line and tissue dependent responses, a CPS was performed using PARP inhibitor treatment in 326 cancer cell lines. This short-term assay used viability as a read out and allowed cell lines to be classified as responders or non-responders to PARP treatment. Using DepMap data to compare between the two responder types, genes and pathways were identified that characterized PARP sensitivity, with results overlapping with the top hits identified from the pooled CRISPRko screen.
Using a 10-cell line panel of clinically relevant cell lines, a CPS combinational study was performed to validate the pooled CRISPRko screen hits. PARP inhibitor treatment was performed in combination with inhibitors targeting various pathways identified from the pooled CRISPRko screen. Here, synergy scores were profiled between drug combinations, validating hits, and identifying cell line vulnerabilities and potential treatment strategies.
Overall, this study showed the strengths of combining FGS and CPS screening for rapid target validation during therapeutic development. FGS provides an in-depth assessment of potential gene and pathway responders. These can be directly validated using CPS, whilst providing the researcher a rapid exploration of responsiveness in multiple cell line backgrounds to build the profile of the clinical utilization potential of a therapeutic. In a similar fashion utilizing these tools in the opposite order could prove just as fruitful, CPS could be used to identify responder cell types, which can then be interrogated by FGS to provide understanding to the drug MOA.
References
- Blanck M, Budnik-Zawilska MB, Lenger SR, McGonigle JE, Martin GRA, le Sage C, Lawo S, Pemberton HN, Tiwana GS, Sorrell DA, Cross BCS. A Flexible, Pooled CRISPR Library for Drug Development Screens. CRISPR J. 2020 Jun;3(3):211-222. https://doi.org/10.1089/crispr.2019.0066
- Blanck M. Leveraging multiplatform cell-based screening for target ID. 2023. https://horizondiscovery.com/en/resources/webinars/leveraging-multiplatform-cell-based-screening
- Zimmermann M, Murina O, Reijns MAM, Agathanggelou A, Challis R, Tarnauskaitė Ž, Muir M, Fluteau A, Aregger M, McEwan A, Yuan W, Clarke M, Lambros MB, Paneesha S, Moss P, Chandrashekhar M, Angers S, Moffat J, Brunton VG, Hart T, de Bono J, Stankovic T, Jackson AP, Durocher D. CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions. Nature. 2018 Jul;559(7713):285-289. https://doi.org/10.1038/s41586-018-0291-z

